 Cause of Death
Cause of Death
Toxicology
is the science that deals with poisons and their effect on the body. In this
activity you will investigate four possible causes of death, lead or mercury
poisoning, aspirin overdose, and diabetes. On the bases of toxicological
evidence a forensics scientist may be able to decide whether the death is due
to natural causes, an accident, suicide, or homicide.
Mercury Poisoning: Mercury in the body causes damage to the proximal renal tubules
in the kidneys and cause the excretion of large amount of the amino acid glycine
in the urine.
Lead Poisoning: Lead in the body causes
damage to the proximal renal tubules in he kidneys and cause the excretion of
large amount of the amino acid methionine in the urine.
Aspirin Overdose: Aspirin, acetysalicylic acid, can cause poisoning and result in
death, especially in children. Aspirin
is hydrolyzed in the body to produce salicylic acid and acetic acid.
Diabetes: It is uncommon but possible for sudden death to
occur due to diabetes. This is caused when the body is unable to control the
amount of sugar, glucose, in the blood. When blood sugar reaches a high level,
toxic substances are produced, causing sickness and sometimes death. At the same time, large amounts of sugar
are excreted in the urine. This presence of sugar is an indicator of
diabetes.
Purpose: In this lab you will test sample solutions
for the presence of glycine, methionine, salicyic acid and sugar. You will also
test an unknown sample.
Procedure:
Part I Testing for Lead and Mercury using Paper
Chromatography
Touch the paper only at the edges to avoid
contamination
1. On a piece of chromatography paper draw a pencil line 2 cm from and parallel to the bottom of the paper. (don’t use a pen)
2 cm

2.
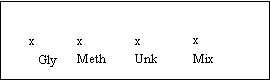
Along
the line place a small “x” 3.0 cm from each side and two additional “x”s evenly
spaced between the outer two. Label
each “x”, Gly, Meth, Unk, Mix
3.
Apply
small spots of the glycine, methionine, unknown, and mixture of glycine and
methionine using a capillary tube. Let each drop dry before applying a second
spot.. Dry with a hair dryer. Keep the spots smaller that .75 cm.
4.
After
the spots are dry, roll the paper into a cylinder with the spots to the
outside. Staple the sides together
without overlap. The lowest staple
should be even with the pencil line at the bottom of the paper.
5.
Add
15 mL of the solvent solution to the chromatography chamber so that the depth
is BELOW the pencil line at the
bottom of the paper when the chromotography paper stands upright on the bottom
of the chamber. Do Not Move the Chamber. Cover with plastic wrap. Leave it in the chamber until the solvent
reaches within an inch of the top of the paper. (About 20 minutes)
6.
Go to Part II and return in 20 minutes
7.
Remove
the paper from the chamber and pencil mark the point at which the solvent
reached. Initial your chromatography
paper and take it to the drying area. A
lab assistant will dry and develop your print.
8.
Go to Part III. Return and pick
up your developed print after completing Part III
9.
Calculate the Rf value for each
spot that appears on your chromatogram by measuring the distance the spot
traveled divided by the distance the solvent traveled.
![]()
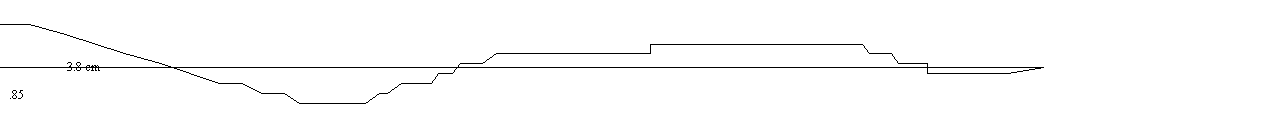 Rf = .85/3.8
Rf = .85/3.8
= .22
Part II Testing for sugar
10.
Place
10 drops of your sample in a small test
tube. To this add 20 drops of
Benedict’s solution. Heat the test tube
in a boiling water bath for 5 minutes.
11.
Repeat
the procedure with your unknown sample.
12.
Record
your observations.
Part III Testing for Aspirin
13.
Place
10 drops of the aspirin solution in a small test tube . To this add 50 drops of FeCl3
test solution.
14.
Repeat
with your unknown and record your results.
Name
____________________________________
Data Table
Part I
1. Sketch your chromotogram.

2. Calculate the Rf values for each spot.
|
Substance |
Distance Traveled |
Rf |
|
Glycine |
|
|
|
Methionine |
|
|
|
unknown |
|
|
|
mixture |
|
|
|
Solvent |
|
xxxxxxxxxx |
3. Describe the results from
the sugar test.
__________________________________________________________________________________________________________________________________________
4. Describe the results from
the aspirin test.
__________________________________________________________________________________________________________________________________________
5. Unknown sample labeled
__#_____ is _____________________.
6. Describe the evidence you used to draw your conclusion.
____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________
Teacher Notes
Solution Preparation:
Glycine -- Add 1.0 grams of glycine to enough water to make 100 mL of solution
Methionine -- Add 1.0 grams of methionine to enough water to make 100 ml of solution
Aspirin -- Mix 60 mL water to 30 mL of ethanol and add 1.0 g of salicylic acid
or dissolve 10 aspirins in a mixture of 60 mL water and 30 mL ethanol. Filter solution
Glucose-- dissolve 10 grams of glucose in 100 mL of water
Benedict’s solution --
FeCl2 solution -- Dissolve 2 grams of FeCl2 · 4 H2O in enough distilled water to make 100mL
Ninhydrin -- Add 0.5 g of ninhydrin in 100 mL of actone
Developing solution -- Add 125 mL of n-butaonl and 125 mL of distilled water and 60 mL of
glacial acetic acid. Shake thorouglhy in a separatory funel and use only the TOP LAYER
Set-up a drying station to collect the chromograms. An adult or student aid should dry the paper, add the ninhydrin, and dry the finished chromogram. The ninhydrin will stain you hands - wear gloves