CHROMATOGRAPHY
One way to separate the substances in a mixture is by use of paper chromatography. In this technique, a drop of the mixture is placed on a piece of filter paper; the filter paper is then dipped like a wick into a solvent, a liquid in which the substances in the mixture will dissolve. The solvent moves along the filter paper by capillary action. When the solvent reaches the mixture, each of the substances in the mixture dissolves and moves along the filter paper also. The dissolved substances move along the filter paper at different rates due to differences in solubility of each substance in the mixture and the size of the molecules that make up the different substances. These differences separate the substances in the mixture. Each substance appears as a separate spot of color on the filter paper.
Question: Are the inks in pens mixtures or pure substances?
Hypothesis:___________________________________________________________________________________________________________________________________
Testing Your Hypothesis:
Chromatography
1. Referring to Figure 1, use scissors to cut a 1 cm strip from the outer edge of a piece of
filter paper to its center.

Figure 1.
2. Fold the strip along the solid, uncut line so that the strip is perpendicular to the surface of the filter paper, as shown in Figure 2.
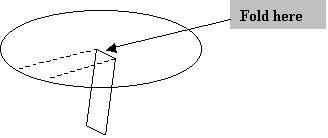
Figure 2
3. Evenly space about the center of the prepared piece of filter paper a small dot of
ink from the 3 different brands of ink pens provided, as shown in Figure 3.
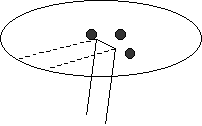
Figure 3
4. Place the filter paper over the top of a 250 mL beaker filled with enough water so that
the bottom of the cut strip of filter paper is submerged to a depth of about 1 cm.
5. The water will move up the strip and across the filter paper by capillary action. This
process is rather slow. Allow the water to move to about 1 cm from the outer edge of
the filter paper.
Data
1. Sketch and label your resulting chromatography separation below.
2. Obtain an unknown ink sample. Record the ink sample ID below. Using your lab data, identify the pen number from which your sample came.
Sample ID ____________ Pen Number ___________
Analysis and Conclusions
1. Was your hypothesis supported or rejected by your data? Explain.
2. Are all inks of the same color composed of the same substances? Explain.
3. Are all the inks you tested water soluble? Explain.
4. If an ink is not water soluble, how could you determine if that ink were a mixture or a
pure substance?