Titanium Anodizing
In a galvanic or voltaic electrochemical cell, the
spontaneous reaction occurs and electrons flow from the anode (oxidation)
to the cathode (reduction). In an electrolytic cell, a non-spontaneous
reaction occurs using energy supplied by an external source. Electrons are
forced from the external circuit at cathode to produce the reduction. The cathode
is the negative electrode and is the black connection on the
power supply. Electrons are drawn into the external circuit at the anode to produce
the oxidation. The anode is the positive electrode and is the red
connection on the power supply.
Some metals form an oxide coating on the surface the
metal as the metal is electrolytically oxidized. Since this oxidation takes
place at the anode, the process is called anodizing. Aluminum metal is commonly
anodized.
2 Al(s) +
3 H2O (l) ® Al2O3
(s) + 6 H+1 (aq) + 6 e-
The
layer of aluminum oxide bonds tightly to the metal and can be used to bond
different dyes to the surface. Many types of sports equipment and furniture
have a durable colored surface of dyed anodized aluminum.
Titanium metal can be oxidized (anodized) by the
following reaction.
Ti(s) +
2 H2O (l) ® TiO2
(s) + 4 H+1 (aq) + 4 e-
The
oxide coating on the titanium, however, acts as a diffraction grating and
separates white light into its colors so that no dyeing is necessary. The color
of the surface depends on the thickness of the oxide layer on the surface,
which is control by the voltage used during the anodizing process.
A typical electrolytic cell is used.
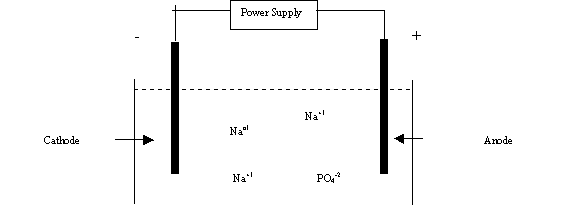
![]()
A stainless steel cathode is used in
the cell. Water is reduced at the cathode.
2 H2O (l) +
2 e- ® H2
(g) + 2 OH-1 (aq)
The
hydrogen gas escapes from the solution and the OH-1 ions enter the
solution. The OH-1 ions react with the H+1 ions produced
at the anode to form water.
Questions:
- Which color lead should
be attached to the cathode?
- Which color lead should
be attached to the anode?
- Write an overall
balanced equation for the reaction occurring the electrolysis.
Sodium
phosphate, Na3PO4, is added to the solution as the
electrolyte so the solution is electrical conducting.
In this experiment, you will design
and anodize a pair of earrings.
Procedure:
Safety glasses
must be worn during this experiment.
1.
Cut the titanium wire to the desired length. Shape with a pair of pliers.
Decide the color pattern you want to create on the wire.
2.
Connect anodizer unit to the step up transformer. Attach the red and black
leads to the anodizer.
3.
Place about 80mL of 5% Na3PO4 solution into a 100mL
beaker.
4.
Bend a small piece of stainless steel so that it will hang over the edge of the
beaker into the solution.
5.
Attach the black lead from the anodizer to the stainless steel electrode.
6.
Use a pair of tweezers to hold the wire. Dip the wire in the multi-etch
solution for a couple of minutes. The multi-etch roughens the surface of the
titanium so the oxide coating adheres better. Remove the wire and rinse the
wire with water. Avoid handling the wire with your fingers to keep from getting
oil from your skin on the wire.
7.
Attach the red lead from the anodizer to the titanium wire.
8.
The voltage determines the color that is formed. The following dial setting on
the anodizer give the correspond color.
9.
You can start at the highest setting to be used and work your way down as you
dip more of the metal into the solution. A lower setting will not anodize over
a higher setting color. You can protect
areas with tape to prevent them from being anodized at a particular setting.
10.
Set the anodizer to the highest setting to be used. Dip the part of the wire to
be anodized at that setting into the solution for about 15-30sec. Be
careful not to let the pin touch the cathode at anytime during the plating
process. Remove the wire
from the solution.
11.
Set the anodizer to the next setting to be used. Dip the part of the wire to be
anodized at that setting into the solution for about 15-30sec. Remove the wire
from the solution.
12.
Set the anodizer to the final setting to be used. Dip the part of the wire to
be anodized at that setting into the solution for about 15-30sec. Remove the
wire from the solution.
13.
Rinse the wire with water and dry the wire.
14.
Turn off anodizer.
15.
Follow your instructor’s directions about coating the wire with a clear acrylic
spray. Attach the earring hangers.
Titanium
Anodizing
Setup Sheet
6 100mL beakers labeled 5% Na3PO4
6 Anodizing units
6 Step-up transformers
6 Pairs of wire leads (one black and one
red) with alligator clips at both ends
6 Tweezers (metal)
6 100mL plastic beakers labeled
Multi-etch.
6 100mL beakers labeled Rinse water
6 Wash bottles
1
liter 5% Na3PO4
Pliers
Titanium
wire
Multi-etch
solution
Acrylic
spray
Titanium Anodizing
Instructor’s Notes
1.
The balanced equation is obtained by
Ti(s) +
2 H2O (l) ® TiO2
(s) + 4 H+1 (aq) + 4 e-
2 [ 2 H2O (l) +
2 e- ® H2
(g) + 2 OH-1 (aq) ]
so
the electrons gained and lost are equal.
Ti(s) +
6 H2O (l) ® TiO2
(s) + 2 H2 (g) 4
H+1 (aq) + 4 OH-1 (aq)
Ti(s) +
6 H2O (l) ® TiO2
(s) + 2 H2 (g) 4
H2O (l)
Final Eqn: Ti(s) +
2 H2O (l) ® TiO2 (s)
+ 2 H2 (g)
2.
Earrings are usually shaped first then anodized. This is also true with rings.
While the wire can be shaped after anodizing, care must be taken to protect the
anodized surface and not scratch it. A piece of cloth can be used.
3.
Plastic containers should be used for the multi-etch since it may attack glass.