ELECTROCHEMICAL CELLS
Prelab
NAME:________________________________________________
PERIOD:_______
1. Given the reaction: Al(s) + Cu+2(aq)
®
Al+3(aq)
+ Cu(s)
a. What is being oxidized?_________
b. What is being reduced?_________
c. What is the oxidizing agent?_________
d. What is the reducing agent?_________
e. Write the half-cell reaction for the
oxidation.
f. What is the standard voltage for this
half-cell reaction?_________
g. Write the half-cell reaction for the
reduction.
h. What is the standard voltage for this
half-cell reaction?_________
i. Write the balanced equation for the
cell reaction.
j.
What is the standard cell voltage for the reaction?_________
k. Is this reaction spontaneous under standard
conditions?_________
2. Given the reaction: Ag (s) + H+1(aq)
®
Ag+1(aq)
+ H2 (g)
a. Write the balanced equation for the
cell reaction.
b.
What is the standard cell voltage for the reaction?_________
c. Is this reaction spontaneous under
standard conditions?_________
d. Is this in agreement with what you
would predict based on the position of the Ag on the Activity Series for metals
and its ability to replace hydrogen? Explain your answer.
ELECTROCHEMICAL CELLS
The
potential difference or voltage of an electrochemical cell depends on the
difference in the electron attracting ability of the two half-cells. The
half-cell with the greater electron attracting ability will be the reduction
half-cell and is designated the cathode. The half-cell with the lower electron
attracting ability will be the oxidation half-cell and is designated the anode.
Electrons leave the electrochemical cell from the anode, flow through the
external circuit, and enter the electrochemical cell at the cathode. The
external circuit may contain a voltmeter to measure voltage and an ammeter to
measure current. The circuit is completed using a salt bridge, which maintains
electrical neutrality in the two half-cells. Ions in the salt bridge migrate into
the two half-cells to balance the charge of any ions lost or produced in the
half-cells.
A
diagram of a typical electrochemical cell is shown below.
External Circuit Zn Electrode Cu Electrode 1.00M Cu(NO3)2 1.00M Zn(NO3)2
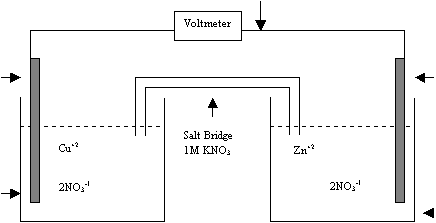
Cathode
Reaction Cu+2(aq) +
2 e- ® Cu(s) Anode
Reaction Zn(s) ® Zn+2(aq) +
2 e-
In a standard cell, the concentration of
the dissolved compounds is 1.00M. A table the standard reduction potentials
provides the half-cell potentials:
Cu+2(aq) + 2
e- ®
Cu(s) Eo
= +0.34V
Zn+2(aq) + 2
e- ®
Zn(s) Eo
= -0.76V
Under standard conditions, the more
positive half-cell has the greater electron attracting ability and will be the
reduction and the cathode reaction. The less positive half-cell has the lower
electron attracting ability and will be the oxidation and the anode reaction. The
anode reaction is reversed and the sign of the half-cell voltage changed. Each
half-cell reaction is multiplied by an integer so the number of electrons lost
equals the number of electrons gained. These half-cell reactions are added to
give the overall balanced equation. The
half-cell voltages are not multiplied by the integers but are simply added
algebraically.
Cathode Cu+2(aq) + 2
e- ®
Cu(s) Eo
= +0.34V
Anode Zn(s) ® Zn+2(aq) + 2
e- Eo
= +0.76V
Overall Cu+2(aq) + Zn(s) ®
Cu(s) + Zn+2(aq) Eocell
= +1.10V
In the cell diagrammed above:
a.
In which
direction do the electrons flow in the external circuit? Why?
b.
What
happens to the mass of the copper electrode? Why?
c.
What
happens to the mass of the zinc electrode? Why?
d.
What happens
to the concentration of the Cu+2 ions in solution? Why?
e.
What
happens to the concentration of the Zn+2 ions in solution? Why?
The
salt bridge is a critical component of the electrochemical cell since it
provides ions that migrate into the two half-cell compartments to maintain
electrical (charge) neutrality. As the cathode reaction occurs, there will be a
surplus of NO3-1 ions in that compartment. K+1
ions migrate out of the salt bridge to balance these NO3-1
ions. As the anode reaction occurs, there will be a surplus of Zn+2
ions in that compartment. NO3-1 ions migrate out of the
salt bridge to balance these Zn+2 ions. A very water-soluble ionic
compound that will not react with the compounds in either half-cell is chosen
for the salt bridge. NaNO3 and NH4NO3 are also
used. A high concentration of ions is used in the salt bridge so that the salt
bridge is not easily depleted of ions otherwise the ions from the two
half-cells will start to migrate through the bridge.
On
a voltmeter, the black terminal is the negative terminal and the terminal where
electrons leave the electrochemical cell and enter the meter. If the voltage on
the meter reads a positive value when the black terminal is connected to an
electrode, that electrode is the anode since the anode half-cell involves
oxidation and releases electrons to the external circuit. The red terminal is
the positive terminal and the terminal where electrons leave the meter and
enter the electrochemical cell. If the voltage on the meter reads a positive value
when the red terminal is connected to an electrode, that electrode is the
cathode since the cathode half-cell involves reduction and gains electrons from
the external circuit. You will sometimes hear the expression, reduce-red-cats,
to help you remember the connection.
In this experiment, you will:
a.
Create a
series of electrochemical cells.
b.
Measure the
voltage of the cells.
c.
Determine
which half-cell is the anode and cathode.
d.
Determine
the order in the reduction potentials of the half-cells.
e.
Compare the
experimental voltages to the theoretical voltages calculated from a table of
standard reduction potentials.
Procedure:
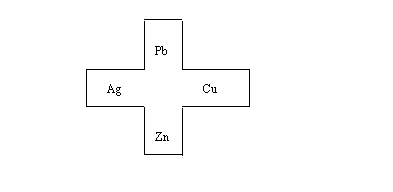
1. Obtain a 9cm piece of filter paper
that has been cut to make a cell template.
2. Write on each sector the atomic symbols
of the metals: Ag, Zn, Cu, and Pb.
3. Place the filter paper on a plastic
sheet and place a piece of the metal on the corresponding
section of the paper.
4. Place 2 drops of a 1M solution of the
corresponding metal ion (AgNO3, Zn(NO3)2,
Cu(NO3)2,
Pb(NO3)2 solution on the paper at the edge of each
metal piece so that each metal is in contact
with its solution.
5. Drop 1M KNO3 into the
middle of the filter paper to form a salt bridge. Be sure that the
KNO3 solution soaks outward and contacts all the other
solutions.
6. Use a voltmeter and measure the
voltage difference between every combination of two
different metals. Touch the probe to each combination of metals. Be sure
make good contact
with the metal. Arrange the probes to achieve positive voltage values.
7. Record your cell voltages.
8. Decide which metal is the cathode and
which metal is the anode.
9. Calculate the theoretical voltages for
each cell.
10. Arrange the metals in order of
strength as a reducing agent from high to low.
CBL
Instructions Using the CBL in the Multimeter Mode:
1. Plug the voltage probe into Channel 1
of the CBL.
2. Attach a short piece of Nichrome wire
to each lead.
3. Turn on the CBL unit and press the MODE
button to switch the CBL to Multimeter mode. It
should be reading voltage. Check to see if there is a mV or V to the right of the center display.
Check to see if Channel 1 and V are displayed in the upper left
corner.
4. Touch the Nichrome wire to each
combination of metals. Be sure make good contact with the
metal.
5. When the meter gives a steady reading,
record the voltage from the CBL.
6. Decide which metal is the cathode and
which metal is the anode.
7. Calculate the theoretical voltages for
each cell.
8. Arrange the metals in order of
strength as a reducing agent from high to low.
II. CBL Instructions Using the CHEMBIO
Program:
1. Prepare the voltage probe.
a. Plug the voltage probe into Channel 1 of the CBL.
b. Attach a short piece of Nichrome wire to each lead.
c. Connect the CBL System to the TI-82 calculator using the
unit to unit link cable. Push the
cable in firmly
at both ends.
2. Turn on the CBL
unit and calculator. Press [PRGM] and select CHEMBIO. Press [ENTER]. (CHEMBIO may be under the APPS menu on the
TI-83+). “Prgm CHEMBIO” appears. Press [ENTER].
“ Vernier Software-Biology and Chemistry with the CBL” appears. Press [ENTER]
again to go to the MAIN MENU. If the CBL and the calculator are not
turned on and the link cable pushed in firmly at both ends, the message “ Link
Error” will appear. Be sure that the link cable is firmly pushed into the CBL
and calculator and press the On/Halt button on the CBL. Press [ENTER]
again to go to the MAIN
MENU.
3. Set up the
calculator and CBL for the voltage probe.
a. Select SET UP PROBES from the MAIN MENU. Press [ENTER].
b. Enter “1” as the number of probes. Press [ENTER].
c. Select VOLTAGE from the SELECT PROBE menu. Press [ENTER].
d. Enter “1” as the channel number. Press [ENTER].
4. Set up the
calculator and CBL for data collection.
a. Select COLLECT DATA from the MAIN MENU. Press [ENTER].
b. Select MONITOR INPUT
from the DATA
COLLECTION menu. Press [ENTER].
No
data will be stored.
c. Prepare the filter
paper and metals as directed above.
d. Touch the Nichrome wire to each combination of metals. Be sure make
good contact with
the metal.
e. When the meter gives a
steady reading, record the voltage from the CBL.
f. If the voltage is negative,
reverse the leads and record the voltage.
g. Decide which electrode
is the cathode and anode.
h. Calculate the
theoretical voltages for each cell.
i. Arrange the metals in
order of strength as a reducing agent from high to low.
j. Press + to QUIT monitoring data. Select QUIT
to exit the program.
This experiment is adapted from A Small-Scale Electrochemical Cell and An Electrochemical Series from Cell Data
in Chemtrek by Stephen Thompson,
Allyn and Bacon, 1990, p. 311-315.
ELECTROCHEMICAL CELLS
NAME:__________________________________________
COURSE:___________
LAB
PARTNER:___________________________________ PERIOD:___________
DATA TABLE
|
Cell |
Cathode |
Anode |
Experimental Voltage |
Theoretical Voltage |
|
Zn-Pb |
|
|
|
|
|
Zn-Cu |
|
|
|
|
|
Zn-Ag |
|
|
|
|
|
Pb-Cu |
|
|
|
|
|
Pb-Ag |
|
|
|
|
|
Ag-Cu |
|
|
|
|
Calculations:
For each of the cells above: write the
oxidation half-cell reaction with its standard voltage, write the reduction half-cell
reaction with its standard voltage, write the balanced cell reaction with the
cell voltage. Include the physical states.
Questions:
1. Why do your experimental voltages
differ from the theoretical voltages?
2. Arrange the metals in order of
strength as a reducing agent from high to low (left to right).
3. What is the relationship between the
strength of the metal as a reducing agent and its standard
reduction potential (be specific about the sign of the standard
reduction potential)?
ELECTROCHEMICAL CELLS
SETUP
SHEET
10 Vials
with a small piece of Zn, Pb, Ag, Cu
10 Sheets
of plastic (overhead transparency sheets will work)
10 Sets
of Berol dropper bulbs filled with 1M solutions of:
Zn(NO3)2, Pb(NO3)2,
AgNO3, Cu(NO3)2, KNO3
10 Wooden
racks to hold the droppers
10 Boxes
Kimwipes
70 9
cm filter paper cut in the form of the template.
10 CBL
with voltage probes and link cables
10 CBL
voltage adapters
20 Short
pieces of Nichrome wire
100ml 1M
Zn(NO3)2, Pb(NO3)2, AgNO3,
Cu(NO3)2, KNO3
ELECTROCHEMICAL CELLS
Instructor’s
Notes
|
Cell |
Cathode |
Anode |
Experimental Voltage |
Theoretical Voltage |
|
Zn-Pb |
Pb |
Zn |
+0.442 |
+0.63 |
|
Zn-Cu |
Cu |
Zn |
+0.984 |
+1.10 |
|
Zn-Ag |
Ag |
Zn |
+1.40 |
+1.56 |
|
Pb-Cu |
Cu |
Pb |
+0.624 |
+0.47 |
|
Pb-Ag |
Ag |
Pb |
+0.907 |
+0.93 |
|
Ag-Cu |
Ag |
Cu |
+0.401 |
+0.46 |
1. Good contact with the metals is
important. The student may need to add an additional drop of the ion solution
if the paper starts to dry out. Buffing off the oxide coating on the metals
will help. Use flats pieces of zinc and copper plate rather than zinc and
copper shot. Flatten the silver wire so that it will make better contact with
the solution.
2. Do not add so much KNO3 that it starts
to dilute the other solutions. Do not add so much of the metal ion solutions
that they run into the other sections.
3. Analog or digital volt meters or
multimeters can be used. Use the smallest scale that will cover 0-2V.
4. If a full size cell is set up as a
demonstration, a salt bridge can be made using a rolled-up piece of filter paper
soaked in KNO3 and folded so it makes contact with the solutions.