Electrolysis – Electroplating of Metals
In a galvanic or voltaic
electrochemical cell, the spontaneous reaction occurs and electrons flow from
the anode (oxidation) to the cathode (reduction). In an electrolytic cell, a
non-spontaneous reaction occurs using energy supplied by an external source.
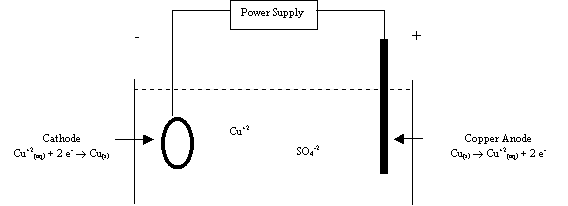
Electrons are forced from the external circuit at cathode to produce the
reduction. The cathode is the negative electrode and is the black connection on
the power supply. Electrons are drawn into the external circuit at the anode to
produce the oxidation. The anode is the positive electrode and is the red
connection on the power supply. Any electrochemical cell can be galvanic or
electrolytic depending on the direction in which the reaction is proceeding.
For instance, a car battery is a galvanic cell when it is discharging and
providing energy and is an electrolytic cell when it is recharging and using
energy. Electrolytic cells are commonly used in industry to produce aluminum,
silver-plate dinnerware, gold-plate jewelry, and chrome-plate car bumpers.
In an electrolytic cell used for
electroplating, the object to be plated is used as the cathode. Metal cations
are reduced at the cathode and a layer of metal is deposited on the surface of
the object. The anode is usually made of the same metal as the one being plated
at the cathode. A concentrated solution of a very water-soluble ionic compound
of metal being plated is used in the cell compartment as the electrolyte. This
makes the solution very conductive and allows the plating to occur relatively
quickly. The rate of plating and the amount of metal plated depend on the
electrical current, in amps, and the time. A cell for plating copper is shown
below,
![]()
Questions:
1.
What
happens to the mass of the anode as the electroplating process occurs?
2.
What
happens to the mass of the cathode as the electroplating process occurs?
3.
What
happens to the [Cu+2] in the solution as the electroplating process
occurs?
4.
Write
the anode and cathode reactions if a zinc anode and ZnSO4 solution
are used.
In
order to get good adhesion between the object being plated and the metal being
deposited on it, the surface of the object must be very clean and free of any
metal oxide coating and other materials such as grease or oil. This may be
accomplished by buffing the object with steel wool or by dipping the object
briefly in an acid solution followed by rinsing with water. After cleaning, the
object should not be handled with your fingers since the oil on your fingers
may interfere with the plating process.
In this experiment, a copper ring
will be copper plated and a copper pin will be zinc plated.
Procedure:
Safety glasses
must be worn during this experiment.
Part 1: Copper Plating the Ring
1.
Fill a 50x100mm crystallizing dish with the copper plating solution to within
an 1/8inch from the top (@210mL). This solution
contains sulfuric acid, is corrosive, and must be handled carefully. Clean up
any spills immediately
2.
Bend a piece of copper sheeting so that it will hang over the edge of the dish
and extend into the solution. This will be the anode and does not have to be
cleaned.
2.
Carefully dip the ring into a beaker containing 6M HCl until it looks like the
oxide coating has been removed (about 30sec).
3.
Remove the ring with tweezers and rinse the ring with water. Handle the ring
with tweezers or with paper towels to avoid getting oil on the surface.
4.
Attach the black alligator clip to the ring. The clip should just hold onto the
edge of the backside of the ring near the solder joint. Attach the red
alligator clip to the copper anode. The
power should be turned on before the ring is lowered into the solution.
5.
Lower ring into the solution. The ring should be completely under the surface
of the solution and held close to the edge of the dish. Plate the ring for
about ten minutes or until and even coating has been created. This may take
less than 10min. Turn the ring around during the plating process so the copper
plates evenly on the outer surface of the ring.
6.
Remove the ring from the solution and rinse with water. Carefully dry the ring.
Follow your instructor’s directions about coating the ring with a clear acrylic
spray.
Part 2: Zinc Plating the Pin
1.
Fill a 50x100mm crystallizing dish with 1.0M ZnSO4 solution to
within an 1/8inch from the top (@210mL).
2.
Bend a piece of zinc sheeting so that it will hang over the edge of the dish
and extend into the solution. This will be the anode and does not have to be
cleaned.
2.
Carefully dip the pin into a beaker containing 6M HCl until it looks like the
oxide coating has been removed (about 30sec).
3.
Remove the pin with tweezers and rinse the pin with water. Handle the pin with
tweezers or with paper towels to avoid getting oil on the surface.
4. Attach
the black alligator clip to the pin. The clip should just hold onto the edge of
the pin. Use a point on the ring that will not interfere with the design on the
ring. The bottom edge may be the best point. Attach the red alligator clip to
the zinc anode. The power should be
turned on before the pin is lowered into the solution.
5.
Lower pin into the solution. The pin should be completely under the surface of
the solution and held close to the edge of the dish. Plate the pin for about
ten minutes or until and even coating has been created. This may take less than
10min. Keep the front surface pointed towards the anode during the plating
process so the zinc plates mainly on that surface of the pin. Some zinc will
plate on the back surface but this will be minimal if the pin is held close to
the side of the dish.
6.
Remove the pin from the solution and rinse with water. Carefully dry the pin.
Follow your instructor’s directions about coating the pin with a clear acrylic
spray.
This experiment is modified from Activity 6.4 Electroplating
a Copper Ring from Art in Chemistry;
Chemistry inArt,by Greenberg and Patterson, Teacher Ideas Press, 1998, pp
165-167.
Electrolysis – Electroplating of Metals
Setup Sheet
5 50x100mm glass crystallizing dishes
labeled copper plating
5 50x100mm glass crystallizing dishes
labeled zinc plating
2 10amp variable transformers
2 6-outlet power strips with circuit
breaker and reset features
10 6/12 volt trickle charge motorcycle
battery rechargers
10 Pairs of wire leads (one black and one
red) with alligator clips at both ends
10 Tweezers (metal)
4 100mL beakers labeled HCl
4 100mL beakers labeled Waste
4 Wash bottles
Copper
plating solution (2 liters)
1.0M
ZnSO4 solution (2 liters)
6M
HCl (500mL)
5 Copper strips (1/2 x 4 inch) for
anodes
5 Zinc strips (1/2 x 4 inch) for anodes
Acrylic
spray
Electrolysis – Electroplating of Metals
Instructor’s
Notes
1.
The ring had already been sized to the individual student and the joint
soldered in a previous activity. The copper for the ring and the pin was
ordered from a jewelry supply house and cut on a metal brake. This avoids sharp
and ragged edges on the ring and pin. The edges may need a little filing if
they are still sharp. The ring was cut from copper strips. The pin was 1x
1.5inch. The pin was cut from copper plate.
2.
The metal for the anodes can be metal sheeting from a lab supply source and was
cut with metal shears.
3.
The copper plating solution came from a commercial plating company and included
brighteners that gave a good shiny coating. The ZnSO4 came from a
lab supply company.
4.
The HCl solution will turn yellow from the dissolved copper ions from the oxide
coating forming a complex ion with chloride ion. This solution should be saved
and reused for this lab even if it is very yellow.
5.
It will save considerable time if a class plating system is set up ahead of
time. One transformer is used for the copper plating and one for the zinc
plating. A power strip in plugged into the transformer. Five motorcycle rechargers
are plugged into the power strip and spaced far enough apart so the students
have sufficient space to work. The black leads from the recharger are attached
to the black alligator wire leads and the red leads from the recharger are
attached to the red alligator wire leads. The crystallizing dishes have the
solutions and the anodes in them already and the anode is attached to the red
leads. Cleaning stations with beakers containing HCl and waste beakers for
rinsing should be set up. The zinc and copper plating stations should be set up
in different parts of the room.
6.
The transformers are set so the battery chargers produce 2V. The voltage output
can be checked using a hand-held volt meter. All the chargers on the same strip
should be producing the same voltage once the transformer is set.
7.
Better plating is achieved if the power is turned on before the object to be
plated in placed in the plating solution. You want a relatively low voltage and
current. A high voltage and current will cause rapid but uneven plating with
metal whiskers coming off the object. You want relatively low current and an
even charge distribution across the object to get even plating. The anode and
cathode should be kept as far apart as possible. The object will plate more heavily
on the surface towards the anode. This is why the ring must be turned around to
get even plating and the front surface of the pin is kept towards the anode.
The object will not plate well were the clip is attached so the clip should be
attached in the least obvious place.
8.
Watch the plating carefully. If the object is left in the solution too long,
you may build up a layer of metal that is thick enough to obscure the surface
design.
9.
The ring and pin must be protected from surface oxidation with an acrylic
spray.
10.
A bas-relief, zinc pin may be created by coating both sides of a 1x1.5 inch
zinc plate with wax. The wax is scraped off the areas that will be etched. The
pin is placed in 6M HCl. The acid will reacted with the exposed zinc. After the
pin has reacted for the desired length of time, the pin is removed and rinsed
with water. The wax is removed by dipping the pin in boiling water.